Technology
Tech could prevent ‘silent threat’ of low blood sugar in Alzheimer’s patients with diabetes
Dementia hinders the ability of patients and caregivers to manage blood sugar levels.
Researchers are redesigning technology to detect low blood sugar in older adults with both diabetes and Alzheimer’s disease.
Scientists are developing user-friendly health information tools and technology to enhance accessibility to older adults with both diabetes and Alzheimer’s disease and their caregivers.
Without numerous finger sticks, these tools and technology will be designed to provide patients, caregivers, and clinicians with glucose metrics needed to diagnose hypoglycemia and identify treatment options.
Older adults with Alzheimer’s and Type 1 or Type 2 diabetes have a high risk of low blood sugar, known as hypoglycemia, which is often undetected. Hypoglycemia can cause dizziness, confusion, mood changes, hospitalisation, and even death. Diagnosis and treatment of hypoglycemia can prevent complications.
Regenstrief Institute Research Scientist April Savoy, PhD, a human factors engineer and health services researcher, said: “Alzheimer’s disease is a progressive disease, and hypoglycemic events can accelerate cognitive decline. That’s why we are focusing on detection of low blood sugar in these older adults with both Alzheimer’s and diabetes.”
Dementia hinders the ability of patients and caregivers to manage blood sugar levels, potentially resulting in hypoglycemic events and further cognitive loss.
Repeated finger sticks to test blood sugar levels, following a prescribed diet and exercising can be difficult for patients with Alzheimer’s. Use of traditional blood sugar monitors may be problematic for individuals with Alzheimer’s as well as caregivers.
The study, ‘Enhancing shared decision-making to prompt and guide individualized care for people with Alzheimer’s Disease and diabetes’ will enrol 75 pairs of older adults with both Alzheimer’s and diabetes, and their family caregivers. Twenty clinicians who see patients with both conditions will also participate.
Research into diabetes and dementia
Other studies have investigated how poor management of diabetes increases risk of dementia. But this new research, implementing a human factors engineering approach, targets older adults who have already been diagnosed with both Alzheimer’s and diabetes.
Dr Savoy and colleagues will investigate unique population dynamics, including how much data about glucose levels are needed, how to acquire them, and what works to enable shared decision-making for all involved, including patients, caregivers, and clinicians.
“We hope to increase awareness and empower patients and caregivers to make individualised, patient-focused decisions about effective treatment options,” said Dr Savoy.
“Our goal is to prevent hypoglycemia for the growing population of older adults with Alzheimer’s disease and diabetes.”
With the use of human-centred design, the authors indicate that they will be able to identify and inform continuous glucose monitoring designs for people with dementia and diabetes, broadening access to continuous glucose monitoring with what they believe is the attainable goal of increasing detection and treatment of the silent threat posed by low blood sugar.
The research is supported by a five-year grant from the National Institutes of Health’s National Institute on Aging.
Research
New drug enhances GLP-1 weight loss without extra added effects, trial finds

Nimacimab significantly enhanced weight loss when combined with GLP-1 therapy without adding side effects, according to mid-stage clinical trial results involving 136 adults.
The experimental drug targets the body’s endocannabinoid system differently from existing weight-loss medications, offering a potential new approach to treating obesity when used alongside drugs such as semaglutide.
Participants given both drugs lost an average of 13.2 per cent of their body weight over 26 weeks, compared with 10.25 per cent for those on semaglutide alone — a statistically significant difference of nearly 3 per cent.
Skye Bioscience’s CBeyond study tested the first-in-class monoclonal antibody, which blocks CB1 receptors involved in appetite regulation and fat storage. The CB1 receptor is part of the endocannabinoid system, which helps control hunger, metabolism and fat accumulation. When these receptors become overactive, they can promote weight gain.
“This is the first clinical study to show that the combination of a CB1 inhibitor and a GLP-1 therapeutic can drive clinically meaningful additional weight loss beyond a GLP-1 drug alone,” said Louis Aronne, past president of The Obesity Society and clinical adviser to Skye Bioscience, the drug’s developers.
“Equally important, although the sample size is small, nimacimab achieved this without neuropsychiatric or additive gastrointestinal adverse events. I believe these results warrant further evaluation of the therapeutic potential of this novel CB1 inhibitor.”
Previous CB1-blocking drugs were abandoned because of psychiatric side effects such as anxiety and depression. Nimacimab has been engineered to stay outside the brain, potentially avoiding the problems that affected earlier drugs targeting this pathway.
In the 26-week trial, adults with overweight or obesity were randomly assigned weekly injections of nimacimab, semaglutide (the active ingredient in Wegovy), both drugs together, or placebo.
When used alone, nimacimab produced modest results — participants lost 1.5 per cent of their body weight compared with 0.26 per cent for placebo, a difference that was not statistically significant. Researchers said exposure to the 200 mg dose was lower than expected, suggesting higher doses may prove more effective.
“The 200 mg monotherapy arm provided important pharmacokinetic insight, showing that lower-than-expected drug exposure may have limited the observed effect and informing the dose-ranging strategy we are developing,” said Puneet Arora, the company’s chief medical officer.
Pharmacokinetic data describe how the body absorbs, distributes, metabolises and eliminates a drug, helping determine optimal dosing.
“At the same time, the combination of nimacimab with semaglutide produced a clinically meaningful additional weight loss that exceeded semaglutide alone, with a favourable tolerability profile even in patients who achieved the highest exposure levels.”
The most striking results came from the combination therapy. All participants receiving both drugs lost more than 5 per cent of their body weight, compared with 85 per cent of those on semaglutide alone. Two-thirds of the combination group lost more than 10 per cent, versus 50 per cent with semaglutide alone.
Importantly, the combination produced a healthier lean-to-fat mass ratio, indicating weight loss came primarily from fat reduction rather than muscle loss — addressing a concern that some GLP-1 drugs may cause skeletal muscle wastage.
Weight loss was still ongoing at the end of the 26-week study, suggesting further reductions could occur with longer treatment.
Safety findings were encouraging across all treatment groups. No neuropsychiatric side effects — such as anxiety, depression or insomnia — were reported with nimacimab, either alone or combined with semaglutide.
Gastrointestinal side effects, a leading cause of discontinuation with GLP-1 therapies, did not increase when nimacimab was added to semaglutide. These typically include nausea, vomiting, diarrhoea and constipation.
The overall discontinuation rate was 27 per cent, with only 3.7 per cent of participants dropping out due to adverse events — most of them in the placebo group.
“Gastrointestinal side effects remain a leading cause of discontinuation with obesity therapies,” said Sean Wharton, director of the Wharton Medical Clinic and a clinical adviser to Skye Bioscience.
“It was notable that nimacimab did not increase GI adverse events while adding clinically meaningful weight loss in combination with semaglutide. In my view, a next study with higher nimacimab dosing is the logical step to fully define its role in clinical practice.”
“With our preclinical data, toxicology safety margin, and PK modelling, we believe we have a path to support higher dosing, and we are evaluating the next stage of development to optimise dosing in potential future clinical trials,” Arora said.
Participants from the Phase 2a study are continuing in a 26-week extension trial, with results expected in early 2026. This will provide data on the longer-term efficacy and safety of the combination approach.
GLP-1 drugs such as semaglutide mimic a hormone that regulates appetite and blood sugar. While highly effective for weight loss, some studies have raised concerns about side effects including kidney injury, skeletal muscle loss and gastrointestinal issues.
A combination therapy that enhances weight loss without compounding side effects could address a major unmet need in obesity treatment, where many patients struggle with the tolerability of current medications.
News
Dementia scan could boost UK diagnosis rates
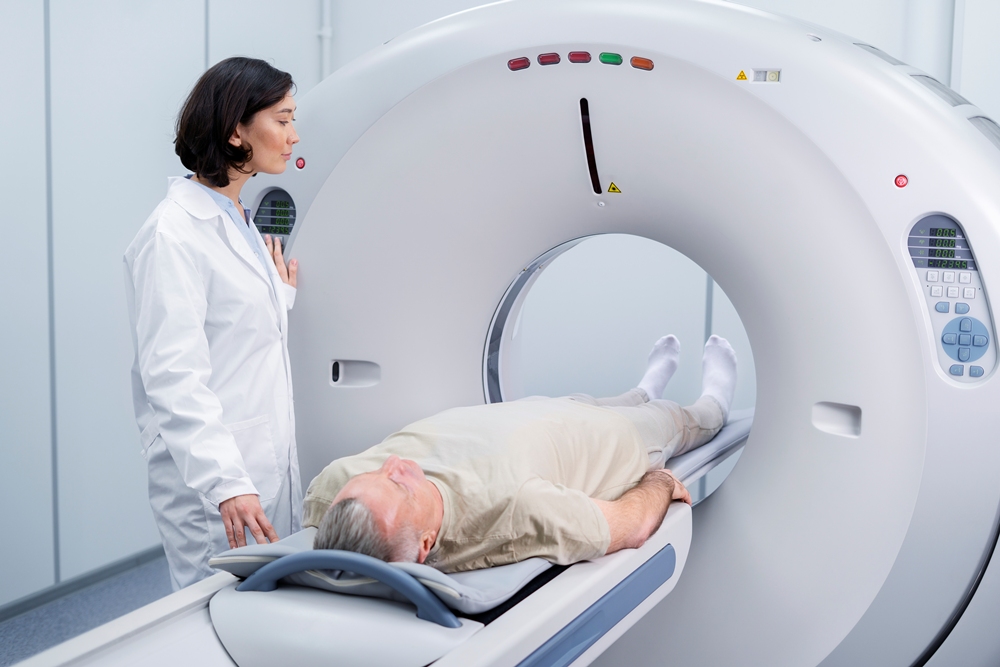
Ultra-fast MRI scans taking under seven minutes could double NHS dementia testing capacity and help boost diagnosis rates across the UK, experts have said.
Scientists have developed a way to cut MRI scan times from 20–30 minutes to less than seven minutes, tackling a key bottleneck in dementia diagnosis, where one in three people never receive one at all.
The technique uses advanced physics to gather more information simultaneously during the scan, reducing total time by almost two thirds. When set-up times are included, the method could at least double capacity across NHS hospitals.
The research was developed by UCL’s Functional Imaging Laboratory and part-funded by Alzheimer’s Society.
Professor Nick Fox, a neurology expert who led the team, said the approach could transform access to vital diagnostic tools.
“This is really exciting. It means that we can make MRI scans — that people deserve to have — much, much more available,” he said.
“One in three people don’t get a dementia diagnosis at all, we estimate. And for those people who do, there are long delays and the recommendation that everybody should have a scan doesn’t happen.
“This could at the very least double the number of MRI scans we can do. There’s no reason why this approach can’t be used across nearly all scanners across hospitals in the UK.”
Current guidelines recommend brain imaging to rule out other causes of cognitive decline or identify the specific type of dementia.
Many patients, however, never receive a scan, and those who do are often given computerised tomography (CT) scans, which provide less detailed images than MRI.
Magnetic resonance imaging (MRI) uses a strong magnet to align protons in the body before knocking them out of position with radio waves.
As the protons return to alignment, sensors detect the released energy to map tissue types and create detailed brain images.
Lying still in the scanner can be particularly difficult for patients with memory problems or claustrophobia. Any movement can blur images, sometimes making them unusable and requiring a repeat scan.
The team tested the faster method on 92 volunteers.
Three neuroradiologists reviewed anonymised scans without knowing which were fast or standard and found the shorter scans were equally reliable for diagnosis.
“What we found was that, when we didn’t tell them which scans were fast or standard, experienced neuroradiologists couldn’t tell the difference,” Prof Fox said.
“The diagnostic ability was just as good, and in some cases slightly better because there was less blurring from people moving. It made much more difference which radiologist looked at your scan, or which day of the week it was.”
Latest NHS data for August showed 66 per cent of people living with dementia were thought to have a diagnosis, meaning one third remain undiagnosed.
Prof Fox said the NHS and government should be more ambitious about not only detecting dementia but identifying the specific cause.
“Saying ‘it’s dementia’ is like saying ‘it’s a rash’. If you went to see your GP, you wouldn’t accept them just saying ‘yes, it’s a rash’. You’d want to know if it’s cancer, eczema, something else,” he said.
“Dementia just means brain failure, it’s not working in the way it should anymore. But the underlying cause could be Alzheimer’s disease, strokes, lots of different things.
“We can only make progress if we give people an accurate diagnosis and a much more timely, rapid one.”
Scans are used alongside cognitive tests to identify the type of dementia. A scan might show past strokes or shrinkage in brain areas linked to memory, such as those affected in Alzheimer’s disease.
Labour’s recent 10-Year Health Plan for England said the UK is “far behind other countries in the levels of CT, MRI and positron emission tomography (PET) scanners for its population”.
The UK has 8.6 MRI scanners per million people — the lowest among comparable nations.
The 2023/24 National Audit of Dementia found that 44 per cent of patients attending specialist memory assessment services had a brain scan, with rates varying from 0–90 per cent depending on the service. In 2021, only 31.8 per cent of scans for suspected dementia were MRI.
MRI scanners cost around £1m. Prof Fox said many hospitals could use the faster method and boost capacity with little or no extra cost, although some may need a software update.
The researchers now plan to work with hospitals to trial the technique, which could also be adapted for other types of body imaging.
News
Weight loss jabs could make cancer scans less effective, study suggests
Weight loss jabs such as Mounjaro and Wegovy could make some cancer scans less effective, leading to unnecessary tests and possible delays in treatment, experts suggest.
The drugs, collectively known as GLP-1s, have helped millions lose up to a fifth of their body weight but may alter how tissue appears on PET-CT scans used to diagnose and stage cancers.
The changes could cause healthy tissue to be misinterpreted as potentially cancerous, meaning some patients may face extra investigations and anxiety while awaiting results.
British researchers at Alliance Medical, a leading provider of diagnostic imaging in the UK, identified the issue after noticing unusual patterns in patient scans.
PET-CT scans combine CT and PET imaging, using a mildly radioactive liquid called a tracer to highlight areas where cells are more active than normal. Brighter spots can indicate cancer, though they may also reflect infections or inflammation.
“We noticed unusual uptake in one of our patients on a GLP-1 agonist, which prompted a wider review across our network,” said Dr Peter Strouhal, medical director at Alliance Medical and lead author of the study.
“We found that these altered patterns are increasingly common, yet there is currently no national or international guidance in the UK addressing this emerging issue.”
The review of several PET-CT scans found atypical tracer patterns among GLP-1 users that “could be misinterpreted” as hot spots or potentially cancerous areas.
“Recognising the characteristic uptake associated with GLP-1 agonists helps avoid unnecessary anxiety and interventions, ensuring patients receive the right care, at the right time, without detours or doubt,” Dr Strouhal said.
Researchers said larger studies involving more data are needed before recommendations can be made to change PET-CT scan guidelines for weight loss jab users.
They advised clinicians to consider a patient’s medical history when interpreting results.
It follows earlier US research suggesting GLP-1 drugs could interfere with breast-cancer chemotherapy medicines.
That study tracked hundreds of women with early-stage triple-negative breast cancer during and after treatment.
Twenty-five were already taking GLP-1s alongside other diabetes drugs and continued doing so while undergoing chemotherapy.
Two years later, 28 per cent of the women on GLP-1s had fully responded to treatment and were clear of cancer, compared with 63 per cent of those not using the drugs.
Researchers also found GLP-1s had entered tumour and immune cells in tissue samples from the patients.
“Use of GLP-1 use may need to be carefully considered during breast cancer therapy,” said Dr Bethania Santos, oncologist and researcher at UT Southwestern Medical Center in Dallas, who presented the study at the San Antonio Breast Cancer Symposium.
However, experts at the time said the drugs themselves may not have reduced the effectiveness of chemotherapy.
People with advanced diabetes who require several medicines already have a higher risk of breast-cancer recurrence.
The study did not prove whether weight-loss drugs help or hinder the success of cancer therapy.

 News1 month ago
News1 month agoSweeteners could age the brain by 1.6 years, research suggests

 News2 weeks ago
News2 weeks agoEarly diagnosis can improve ‘dementia journey’, charity says

 News2 months ago
News2 months agoHand wringing may signal advanced dementia

 News1 month ago
News1 month agoOxford builds ‘organs-on-a-chip’ to unlock new heart therapies

 Research6 days ago
Research6 days agoHalf of Brits fear dementia more than any other condition, research finds

 News2 months ago
News2 months agoParticipants sought for ‘groundbreaking’ dementia study

 Insights3 weeks ago
Insights3 weeks agoCycling may lower dementia risk, study finds

 News1 month ago
News1 month agoBrainwave test spots early Alzheimer’s signs years before diagnosis